Tenecteplase is a third generation thrombolytic produced with recombinant DNA technology as a modified form of alteplase. These changes prolongs the half life of tenecteplase and allow greater binding affinity for fibrin than alteplase.
Studies on Tenecteplase v/s Alteplase in AIS
ATTEST Trial (2015)
It is randomised, open label, blinded end point done to assess the effi cacy and safety of tenecteplase (0.25 mg/kg) versus alteplase (0.9 mg/kg) within 4·5 h of stroke onset in a population not selected on the basis of advanced neuroimaging, and to use imaging biomarkers to inform the design of a definitive phase 3 clinical trial.The study found that there was no significant difference was noted for percentage of pneumbra salvaged 68% for tenecteplase and 68% for alteplase group. Serious adverse events didnot differ in both groups.
NOR-TEST (2017)
It is randomised, open label, blinded end point done in 13 stroke units in Norway. It is done to investigate safety and efficacy of tenecteplase (0.4 mg/kg) v/s alteplase (0.9mg/kg) in patients with acute stroke presenting within 4.5h (n=1100. Primary outcome defined as mRS score 0-1 at 3 months was achieved in 64% in tenecteplase group and 63% in alteplase group.Serious adverse events were similar in both groups. Study concluded that tenecteplase was not superior to alteplase and showed similar safety profile.
ACT Trial (2022)
It is a multicentre, open-label, parallel-group, registry-linked, randomised, controlled trial (AcT), patients (n=1600)were enrolled from 22 primary and comprehensive stroke centres across Canada, was done to determine whether tenecteplase (0.25mg/kg) increase reperfusion compared to alteplase(0.9mg/kg). Study found that 36.9% in tenecteplae group and 34.8% in alteplase group had an mRS score of 0-1 at 90-120 days. In safety analysis 3.4% in tenecteplase group and 3.2% in alteplase had 24h symptomatic intracerebral hemorrhage and 15.3% and 15.4% died within 90 days of starting treatment. Study concluded that IV tenecteplase is a reasonable alternative to alteplase for all patients presenting with AIS.
In a pre specified subgroup analysis of ACT Trial author compared outcomes in Large Vessel Occlusion (LVO). 33% of study population had LVO and proximal MCA was most common site of obstruction. Subgroup analysis found an excellent outcome in 33% of teneceteplase and 30% in alteplase patients - a non significant difference. Rates of symptomatic intracerebral hemorrhage (6% tenecteplase, 4% alteplase) and 90-day mortality (20% tenecteplase, 18% alteplase) were similar in the two groups. In patients who received emergent thrombectomy, initial angiographic recanalization did not differ between the tenecteplase group (9%) and the alteplase group (11%).
TRACE-2 (2023)
In this multicentre, prospective, open-label, blinded-endpoint, randomised controlled, non-inferiority trial was done to establish the non-inferiority of tenecteplase to alteplase in AIS.Adults with an acute ischaemic stroke who were eligible for standard intravenous thrombolysis but ineligible for endovascular thrombectomy were enrolled from 53 centres in China and randomly assigned (1:1) to receive intravenous tenecteplase (0·25 mg/kg, maximum dose of 25 mg) (n=714) or intravenous alteplase (0·9 mg/kg, maximum dose of 90 mg) (n=716).The primary outcome in the modified intention-to-treat population occurred in 439 (62%) of 705 in the tenecteplase group versus 405 (58%) of 696 in the alteplase group (RR 1·07, 95% CI 0·98–1·16). The lower limit of the RR’s 95% CI was greater than the non-inferiority margin. Symptomatic intracranial haemorrhage within 36 h was observed in 15 (2%) of 711 in the tenecteplase group and 13 (2%) of 706 in the alteplase group (RR 1·18, 95% CI 0·56–2·50). Mortality within 90 days occurred in 46 (7%) individuals in the tenecteplase group versus 35 (5%) in the alteplase group (RR 1·31, 95% CI 0·86–2·01).Tenecteplase was non-inferior to alteplase in people with ischaemic stroke who were eligible for standard intravenous thrombolytic but ineligible for or refused endovascular thrombectomy
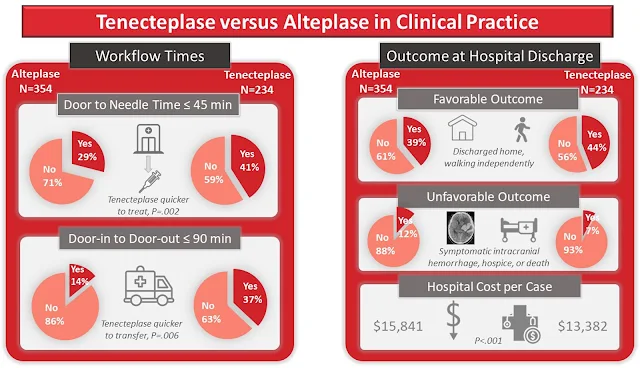
A recent observational cohort study on teneceteplase v/s alteplase in routine clinical practice by Warach et al found that switching to teneceteplase was associated with shorter door to needle time and non inferior clinical outcomes at discharge.
Conclusion
As of now only alteplase has FDA approval for AIS. ASA guidelines states it is reasonable to use tenecteplase (0.25mg/kg, Max:25mg) as alternative to alteplase. Recent studies have shown that teneceteplase is non inferior to alteplase & has similar adverse effects . Ease of administration of tenecetplase as single bolus dose can be beneficial as it requires lesser resource and results in early administration of drug.
Reference
- Mahmood A, Muir KW. Tenecteplase or Alteplase: What Is the Thrombolytic Agent of the Future? Curr Treat Options Neurol. 2022;24(10):503-513. doi: 10.1007/s11940-022-00733-4. Epub 2022 Jul 30. PMID: 35965955; PMCID: PMC9362569.
- https://www.jwatch.org/na56353/2023/07/24/tenecteplase-vs-alteplase-large-vessel-occlusion-stroke
- Bala F et al. Safety and efficacy of tenecteplase compared with alteplase in patients with large vessel occlusion stroke: A prespecified secondary analysis of the ACT randomized clinical trial. JAMA Neurol 2023 Jul 10; [e-pub]. (https://doi.org/10.1001/jamaneurol.2023.2094. opens in new tab)
- Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, Thirunavukkarasu S, Khosravani H, Appireddy R, Moreau F, Gubitz G, Tkach A, Catanese L, Dowlatshahi D, Medvedev G, Mandzia J, Pikula A, Shankar J, Williams H, Field TS, Manosalva A, Siddiqui M, Zafar A, Imoukhuede O, Hunter G, Demchuk AM, Mishra S, Gioia LC, Jalini S, Cayer C, Phillips S, Elamin E, Shoamanesh A, Subramaniam S, Kate M, Jacquin G, Camden MC, Benali F, Alhabli I, Bala F, Horn M, Stotts G, Hill MD, Gladstone DJ, Poppe A, Sehgal A, Zhang Q, Lethebe BC, Doram C, Ademola A, Shamy M, Kenney C, Sajobi TT, Swartz RH; AcT Trial Investigators. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022 Jul 16;400(10347):161-169. doi: 10.1016/S0140-6736(22)01054-6. Epub 2022 Jun 29. PMID: 35779553.
- Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, Schwamm LH, Fisher M, Che F, Dai H, Li D, Li R, Wang J, Wang Y, Zhao X, Li Z, Zheng H, Xiong Y, Meng X; TRACE-2 Investigators. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet. 2023 Feb 25;401(10377):645-654. doi: 10.1016/S0140-6736(22)02600-9. Epub 2023 Feb 9. Erratum in: Lancet. 2023 Apr 1;401(10382):1078. PMID: 36774935.
- Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, Ford I, Muir KW. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015 Apr;14(4):368-76. doi: 10.1016/S1474-4422(15)70017-7. Epub 2015 Feb 26. PMID: 25726502.
- Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, Thommessen B, Amthor KF, Ihle-Hansen H, Kurz M, Tobro H, Kaur K, Stankiewicz M, Carlsson M, Morsund Å, Idicula T, Aamodt AH, Lund C, Næss H, Waje-Andreassen U, Thomassen L. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017 Oct;16(10):781-788. doi: 10.1016/S1474-4422(17)30253-3. Epub 2017 Aug 2. PMID: 28780236.